| Stage at diagnosis | TNM stage | 5-year relative survival rate(%) |
|---|---|---|
| Localized (confined to primary site) | I–II | 52.2 |
| Regional (spread to regional lymph nodes) | III | 25.1 |
| Distant (cancer has metastasized) | IV | 3.7 |
| Unknown (unstaged) | n/a | 7.9 |
| TNM = tumor, node, metastasis. | ||
| Stage/ Tumor Type |
TNM code | Treatment |
|---|---|---|
| Stage IA | Peripheral T1ab, N0 |
Surgical resection (wedge/lobectomy) with or without radiation therapy |
| Stage II | T1, N1, M0 T2, N1, M0 T3, N0, M0 |
Lobectomy, pneumonectomy, segmental, wedge or sleev resection as appropriate Radiation therapy with curative intent for potentially operative in patients with medical contraindications to surgery Adjuvant chemotherapy after curative surgery |
| Stage IIIA | T1, N2, M0 T2, N2, M0 T3, N1, M0 T3, N2, M0 |
Surgery is an option if the tumor is resectable, followed by adjuvant chemotherapy If the tumor is unresectable the patient is given chemoradiation In case the patient is unfit for combined modality therapy, they are given radiation therapy alone |
| Superior sulcus tumors |
T3, N0, M0 T3, N1, M0 |
Radiation therapy and surgery Radiation therapy alone Surgery alone Concurrent chemotherapy with radiation therapy and surgery |
| Chest wall tumor |
T3, N0, M0 T3, N1, M0 |
Surgery alone Surgery and radiation therapy Radiation therapy alone Chemotherapy combined with radiation therapy and/or surgery |
| Stage IIIB | Any T, N3, M0 T4, anyN, M0 |
Surgery followed by chemotherapy if margins are negative Surgery followed by concurrent chemoradiation if the margins are positive and if it is tolerated by the patient |
| Stage IV | Any T, Any N, M1a or M1b |
Depending on the site of metastasis and the size of the tumor, treatment would include surgery, chemotherapy, chemoradiation therapy, radiation therapy or a combination of any or all of these |
| Adjuvant Therapy | |
|---|---|
| Regimen | Duration |
| Cisplatin 50mg/m2 days one and eight Vinorelbine 25mg/m2 days one, eight, 15 and 22 |
Every 28 days for four cycles |
| Cisplatin 100mg/m2 day one Vinorelbine 30mg/m2 days one, eight, 15 and 22 |
Every 28 days for four cycles |
| Cisplatin 75–80mg/m2 day one Vinorelbine 25–30mg/m2 days one and eight |
Every 21 days for four cycles |
| Cisplatin 100mg/m2 day one Etoposide 100mg/m2 days 1–3 |
Every 28 days for four cycles |
| Cisplatin 80mg/m2 days one, 22, 43 and 64 Vinblastine 4mg/m2 days one, eight, 15, 22 then every two weeks after day 43 |
Every 21 days for four cycles |
| Other acceptable cisplatin based regimen | |
| Cisplatin 75mg/m2 on day one Gemcitabine 1,250mg/m2 on days one and eight |
Every 21 days |
| Cisplatin 75mg/m2 Taxotere (docetaxel) 75mg/m2 |
Every 21 days |
| Alimta (pemetrexed) 500mg/m2 on day one Cisplatin 75mg/m2 on day one |
Every 21 days for four cycles |
| For patients with comorbidities or patients not able to tolerate cisplatin | |
| Paclitaxel 200mg/m2 on day one Carboplatin AUC 6 on day one |
Every 21 days |
| Chemotherapy regimens used with radiation therapy | |
| Concurrent chemotherapy/RT regimens | |
| Cisplatin 50mg/m2 days one, eight, 29 and 36 Etoposide 50mg/m2 days 1–8, 29–33 Concurrent thoracic RT (preferred) |
|
| Cisplatin 100mg/m2 days one and 29 Vinblastine 5mg/m2/ five times weekly Concurrent thoracic RT (preferred) |
|
| Paclitaxel 45–50mg/m2 weekly over one hour Carboplatin AUC = 2mg/ml/min over 30 min weekly Concurrent thoracic RT |
|
| Concurrent chemotherapy/RT followed by chemotherapy | |
| Cisplatin 50mg/m2 days one, eight, 29 and 36 Etoposide 50mg/m2 days 1-8, 29-33 Concurrent thoracic RT Followed by Cisplatin 50mg/m2 and Etoposide 50mg/m2 x 2 additional cycles |
|
| Paclitaxel 45–50mg/m2 weekly Carboplatin AUC 2, concurrent thoracic RT Followed by 2 cycles of Paclitaxel 200mg/m2 and Carboplatin AUC 6 |
|
| First-line | |
|---|---|
| Avastin (bevacizumab) + chemotherapy or chemotherapy alone (PS 0-1) | 4–6 cycles, Avastin given until disease progression |
| Erbitux (cetuximab) + vinorelbine/cisplatin (PS 0-2) | 4–6 cycles |
| Tarceva (erlotinib EGFR mutation positive patients) | Given until disease progression (median duration 9.9 weeks*) |
| Second line | |
| Single-agent docetaxel, Alimta, erlotinib | |
| Third-line | |
| Tarceva | |
| Maintenance therapy | |
| Continuation of Avastin after 4–6 cycles of platinum -doublet chemotherapy and Avastin |
|
| Continuation of Erbitux after 4–6 cycles of cisplatin, vinorelbine, and Erbitux | |
| Continuation of Alimta after 4–6 cycles of cisplatin and Alimta for patients with histologies other than SCC |
|
| Initiation of Alimta after 4–6 cycles of first-line platinum -doublet chemotherapy for patents with histologies other than SCC |
|
| Initiation of Tarceva after 4–6 cycles of first-line platinum -doublet chemotherapy |
|
| Initiation of Taxotere after 4–6 cycles of first-line platinum -doublet chemotherapy |
| Oncogene | Mutation Prevalence | Mutation-Predicted Therapeutic Response | Predicted Response Rate |
|---|---|---|---|
| EGFR | Asians: 40% Caucasians: 10-15% |
Sensitive to EGFR TKIs | Tarceva: ~82% Iressa: ~73% |
| KRAS | Asians: 10% Caucasians: 30% |
Resistant to EGFR TKIs | Data not available |
| ALK | 5% | Sensitive to ALK inhibitors, Resistant to EGFR TKIs |
Xalkori (crixotinib): ~57% |
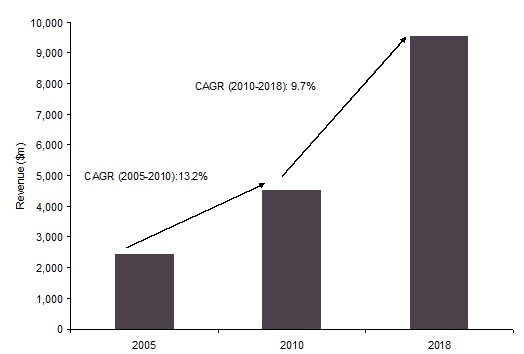
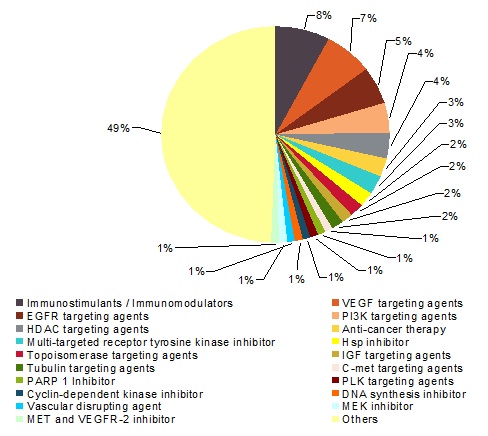
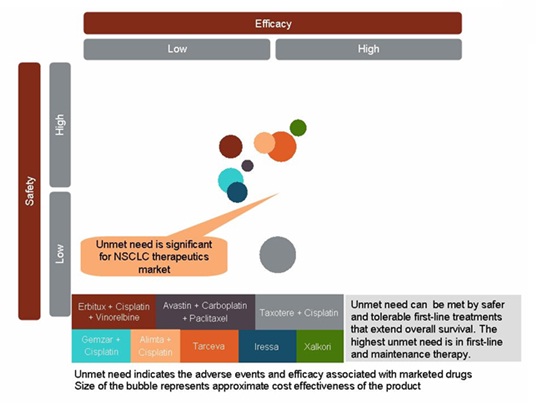
이전
2013.03.04
다음
2013.05.06