[전문가 기고_권병세]
Immune Checkpoint Therapeutics
Byoung S. Kwon, PhD, Eutilex, Co., Ltd.
1. Costimulatory receptor-ligand system
T lymphocytes requires two signals for a proper activation. Signal one is antigen-specific and comes from T cell antigen receptor (TCR) when it binds to a specific antigens, and signal two comes from collectively-called costimulations. These costimulations are cell surface receptor-ligand systems; receptors are on the T cell surfaces and ligands are on the antigen-presenting cells (APCs) or vice versa. The interaction between costimulatory receptors and ligands usually controls the intensity, direction and duration of T cell-mediated immune responses.
The costimulation can be divided into two groups; the receptors that function when the antigen engagement occurs (called “constitutive costimulation” such as CD28 and HVEM) and the ones that are induced upon antigen engagement, and therefore, function in an antigen-specific manner (called “inducible costimulation” such as 4-1BB also known as CD137, and AITR) (Kwon and Weissman, 1989) (Figure 1).
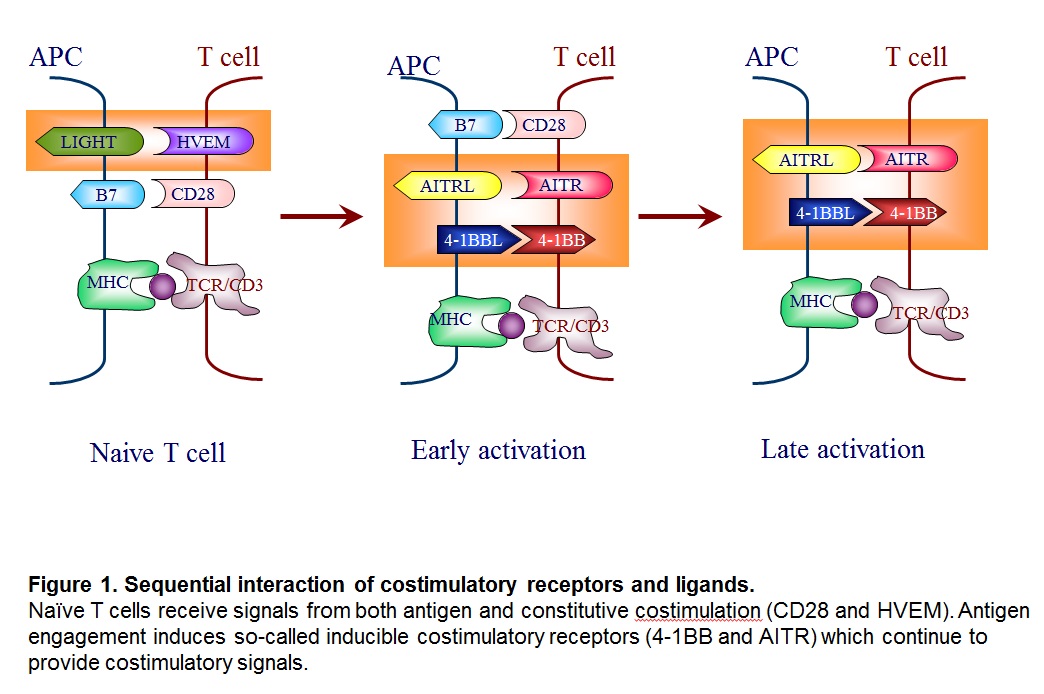
Many costimulatory receptors and ligands have been identified. The first major pathways to be identified and characterized as costimulation were the B7/CD28 and CD40/CD154 pathways. Many additional pathways have been discovered with clear homologous associations with know costimulatory molecules, and the concept of costimulation has spawned a complementary concept of co-inhibition. Together, these pathways have been woven into a view of immunity as a balanced process of activation and resolution. Furthermore, costimulatory molecules have been shown to exist not only on lymphoid but also on parenchymal tissues. Thus, costimulation has grown to be seen as a process by which immune responses occur cognizant of the state of the parenchyma in the maintenance of organism homeostasis (see Figure 2).
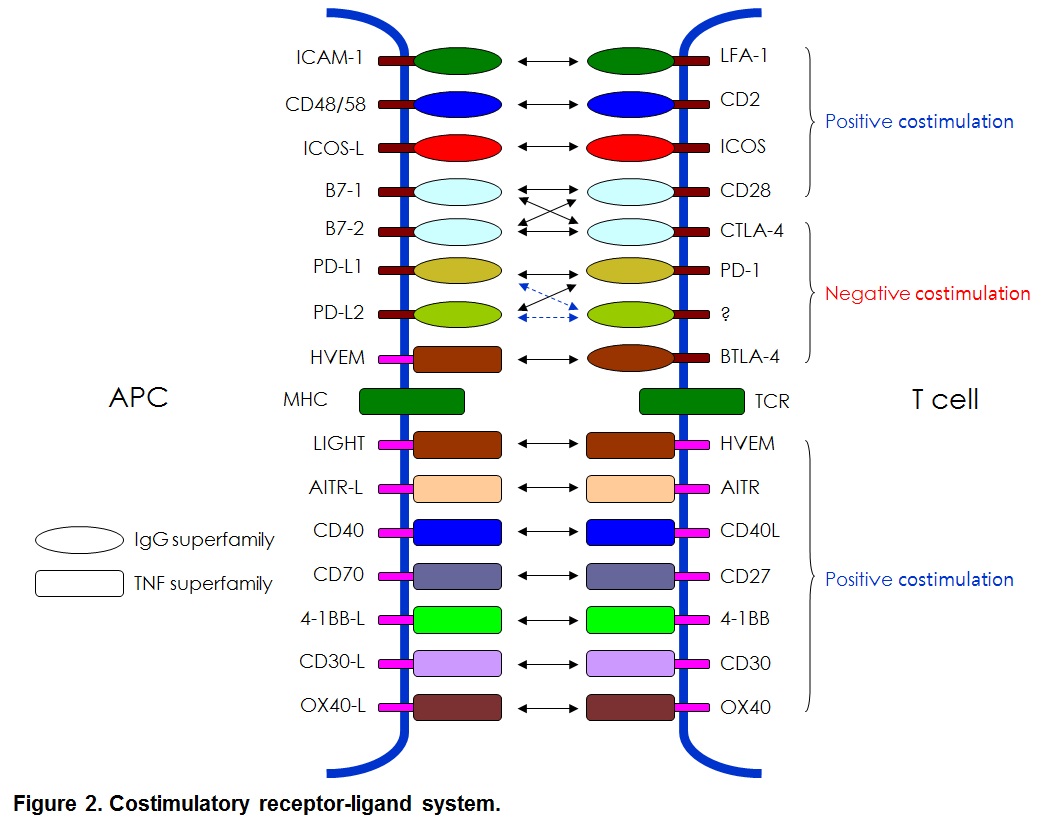
Molecular characteristics identified that these molecules can be divided into Ig supergene family such as CD28, ICOS and PD-1 and TNFR supergene family such as 4-1BB(CD137), OX40, AITR and CD40 (Figure 2). The last decade has seen considerable work in the manipulation on costimulatory molecules to eliminate allograft rejection and cancer treatments.
2. Costimulation-mediated immunotherapy
Manipulation of costimulation can enhance or suppress immune responses. Theoretically, modulation of inducible costimuation can result in antigen-specific enhancement or suppression of immune responses. Suppression of immune response can apply to treat harmful immune responses such as autoimmune diseases. Enhancement of immune responses can be used to treat cancers and viral diseases. Much effort is marking to develop therapeutics at both directions. Current article is, however, focused on development of anti-cancer therapeutics.
Successful treatment of cancer is difficult due to several factors, including immune evasion, defective antigen presentation, production of immune suppressive factors, tolerance and immune deviation, apoptosis of immune competent cells, tumor heterogeneity and metastasis (Vinay et al., 2015). Extensive research over the decades has broadened our knowledge of cancer treatments, and several novel and promising approaches have undergone clinical trials. Among others, inhibitors of immune check point because they block negative regulators of T cell immunity, have shown tremendous potential as an anti-cancer strategy with marked effects on overall survival and quality of life (Pastow et al., 2015). At the molecular level, blockade of inhibitory immune checkpoint leads to recovery of T cell effector function that has been lost due to overt regulatory activity or triggering of inhibitory receptos/ligands.
Among the checkpoint blocking antibodies, the most successful are those directed against CTLA-4 and PD-1: they have shown marked therapeutic potential in the treatment of variety of tumor types (Pardoll, 2012). While CTLA-4 attenuates the early activation of naïve and memory T cells via its interaction with B7-1/B7-2, PD-1 acts via interaction with its ligands, PL-L1 and PL-L2 (Kyu and Pastow, 2014). Although CTLA-4 and PD-1 are both negative immune regulators, they play discreet roles in reducing the strength of ongoing immune responses. Immune checkpoint pathways are currently the subject of intense research. Some immune checkpoint targets are either in the clinic or entering the clinic. Here we discuss not only those immune checkpoint inhibitors that have been approved for cancer therapy but also those that have been formed to possess anti-tumor activity and are currently being evaluated (Figure 3).
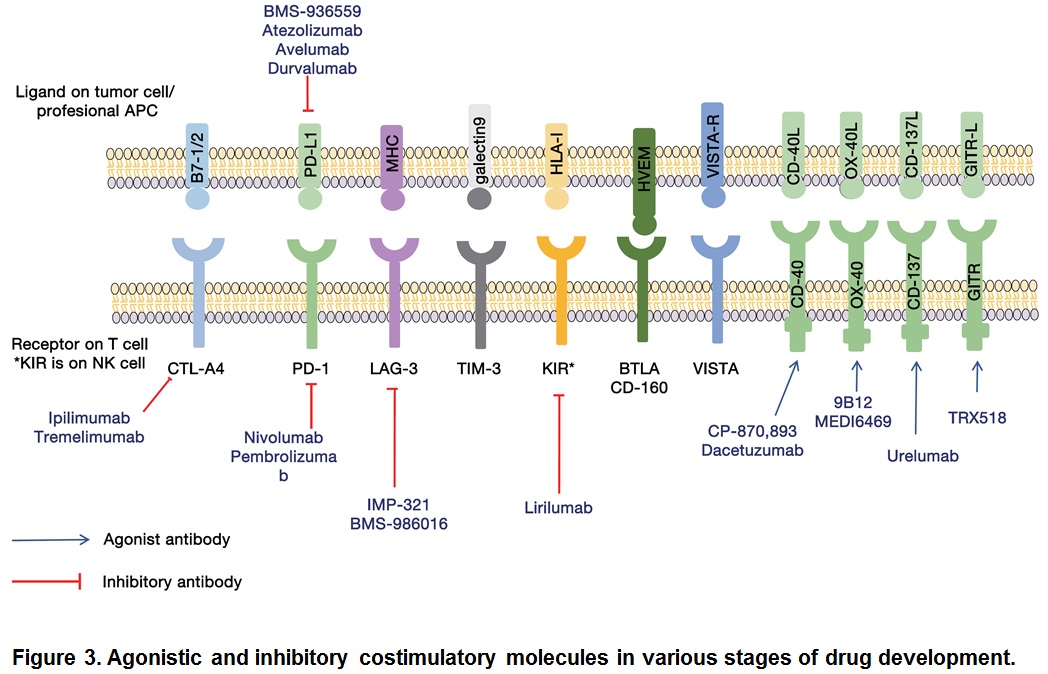
Many clinical trials are being conducted to test the efficacy of combination therapies. Combination of CTLA4 and PD-1/PD-L1 produced a promising outcome (Callahan et al., 2014). When antibodies to PD-1 and CTLA-4 cancer regression were observes in over 80% of patients (Wolchok et al., 2013). Current trials include not only combination of immune checkpoint inhibitors but also among immune checkpoint inhibitors and enhancers such as urelumab and TRX518. We have certainly equipped with many more armamentarium against cancers.
3. Side effects
As with other cancer therapies, immune checkpoint therapies have side effects and toxicities (Gao et al., 2015; Postow et al., 2015) which are major stumbling blocks in the clinic (Weber et al., 2012). These side effects are the results of adverse immune activity leading to mild to severe inflammatory conditions, including dermatitis, colitis, hepatitis, pancreatitis, pneumonitis, and hypophysitis. A number of side effects, mostly immune-related, were noted in ipilimumab-treated patients; they included injury to spleen, liver, and kidney, diarrhea, enterocolitis, dermatitis, and endcrinopathies (Barnard et al., 2012; Wolchok et al., 2010). The effects could be dealt with by stopping ipilimumab therapy and treating with corticosteroids, mycophenolate mofetil or tumor necrosis factor (TNF) –alpha blockade (Weber et al., 2012). In addition, around 10% of patients treated with anti-PD-1 developed severe, sometimes life-threatening, grade 3-4 immune-related toxicities (Weber et al., 2015). Anti-CTLA-4 and anti-PD-1/PD-L1 therapies have also been linked to colitis and pneumonitis (Champiat et al., 2015). Interestingly the immune infiltrates induced by immune checkpoint blockade were found to amplify peritumoral inflammation and have a toxic effect in the region of the tumors. Chronic T-cell exhaustion has also been reported due to PD-1 blockade (Wherry, 2011). Although the side effect of anti-CTLA-4 and anti-PD-1/PD-L1 antibody therapies are similar, their magnitude is generally higher in the case of anti-CTLA-4. However, many of these side effects can be controlled by treatment with immunosuppressive compound such as anti-IL-6 without interfering with their anti-tumor activity. It seems likely that further advances in this field will help overcome these associated side effects and aid in streamlining treatments with these immune checkpoint inhibitors.
4. Other checkpoint therapeutics
Although CTLA-4 and PD-1/PD-L1 are central players in immune checkpoint therapy of cancer, there are many other immunologic pathways that could be targeted. Examples are: immune molecules such as lymphocyte activation gene 3 (LAG-3) (Triebel et al., 1990). T cell membrane protein (TIM3) (Sakuishi et al., 2010), B and T lymphocyte attenuator (BTLA) (Watanabe et al., 2003), adenosine A2a receptor (A2aR) (Zarek et al., 2008), and costimulatory molecules like CD27 (Vitale et al., 2012), AITR (GITR) (Kwon et al., 1999), OX-40 (Curti et al., 2013), and 4-1BB (Sznol et al., 2008; Segal et al., 2014). All of these have shown anti-cancer effects in pre-clinical studies (Figure 3).
References
Barnard ZR, Walcott BP, Kahle KT, et al. 2012. Hyponatremia associated with ipilimumab-induced hypophysitis. Med Oncol. 29:374-377.
Callahan MK, Bendell JC, Chan E, et al. 2014. Phase I/II open-label study of nivolumab (anti-PD-1;BMS-936558, ONO-4538) as monotherapy or combined therapy with iplimumab in advanced or metastatic solid tumors. J Clin Oncol 32:5s (suppl; abstr TPS3114)
Curti BD, Kovacsovics-Bankowski M, Morris N, et al. 2013. OX-40 is a potent immune-stimulating agent in late-stage cancer patients. Cancer Res 73:7189-7189.
Kwon BS, Weissman SM. 1989. cDNA sequences of two inducible T cell genes. Proc Natl Acad Sci USA 86:1963-1967.
Kwon B, Yu KY, Ni J, Yu GL, Jang IK, Kim YJ, Xing L, Liu D, Wang SX, Kwon BS. 1999. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J Biol Chem. Mar 5;274(10):6056-61.
Kyu C, Postow MA. 2014. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 588:368-376.
Pardoll M. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252-264.
Postow MA, Callahan MK, Wolchok JD. 2015. Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974-1982.
Sakuishi K, Apetoh L, Sullivan JM, et al. 2010. Targeting TIM-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207:2187-2194.
Segal NH, Gopal AK, Shailender B, et al. 2014. A phase I study of PF-05082566 (ani-4-1BB) in patients with advanced cancer. J Clin Oncol 32:5s (suppl; abstr 3007)
Sznol M, Hodi FS, Margolin K, et al. 2008. Phase I study of BMS-663513, a fully human anti-CD137 agaonist monoclonal antibody, in patients (pts) with advanced cancer (CA). J Clin Oncol 26: Abstracts no. 15_suppl 3007
Triebel F, Jitsukawa, S, Baixeras E, et al. 1990. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 171:1393-1405.
Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015 Dec;35 Suppl:S185-98.
Vitale LA, He LZ, Thomas LJ, et al. 2012. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin Cancer Res 18:3812-3821.
Watanabe N, Gavrieli M, Sedy JR, et al. 2003. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 4:670-679.
Weber JS, Kahler KC, Hauschild A. 2012. Management of immune–related adverse events and kinetics of response with ipilimumab. J Clin Oncol 30:2691-2697.
Wherry EJ. 2011. T cell exhaustion. Nat Immunol 12:492-499.
Wolchok JD, Kluger H, Callahan MK, et al. 2013. Nivolumab plus ipilimumab in advanced melanoma. N Eng J Med 369:122-133.
Wolchok JD, Neyns B, Linette G, et al. 2010. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomized, double blind, multicenter, phase II, dose-ranging study. Lancet Oncol 11:155-164.
Zarek PE, Huang CT, Lutz ER, et al. 2008. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cell. Blood 111:251-259.
이전
2016.05.02
다음
2016.07.04