(KDDF-201410-08) Phase IB Clinical Study of JPI-289 for the Treatment of Stroke
CNS Disease, Chemical
The aim of this project is to accomplish phase IB clinical trial including evaluation of safety/tolerability and PK/PD profiles of JPI-289, and to proceed phase IIA clinical development of the PARP-1 inhibitor, as a first-in-class drug for the treatment of acute ischemic stroke.
Ultimately, after rapid completion of Proof of Concept (POC, Phase IIA) clinical studies, Jeil Pharmaceutical is planning to license out, co-develop, and co-commercialize JPI-289 with global big companies.
Stroke is a debilitating condition, with the highest death rate as a single organ disease. Incidence is expected to keep increasing over the next 20 years despite the progress in modern medical science and technology. This is largely due to a global increase in the aging population.
No clinically effective therapies currently exist although the stroke incident rate in Korea remains high. Developments of new therapeutic strategies and standards of medical care are imperative.
Currently, treatment for ischemic stroke with cerebral blood vessel occlusion predominantly employs a thrombolytic agent combined with surgery and further agents including anticoagulants and platelet aggregation inhibitors for secondary prevention. Any new therapy for stroke is expected to be highly marketable, as the only thrombolytic agent, tPA (tissue Plasminogen Activator) has been approved by the FDA with limitations.
Jeil Pharmaceutical will develop JPI-289, a PARP-1 inhibitor, on a commercial scale after acquiring Proof-of-Concept data via a combination therapy with tPA in Phase IIA clinical trials. This will be followed by licensing-out and co-development with multinational partners.
A homogeneous patient group will be selected for the tPA co-administration for NDA approval, with the range of subjects and indications further expanded through PMS clinical trials.
A clinical protocol for a phase IB clinical trial of JPI-289 was approved by Ministry of Food and Drug Safety (MFDS) and Institutional Review Board (IRB) of Asan Medical Center, and the MAD (Multiple Ascending Dose) clinical trial in healthy male subjects is currently in progress.
A clinical protocol for a phase IIA clinical trial in stroke patients will be developed by the end of 2015. After acquiring IND/IRB approvals for the phase IIA clinical trial of JPI-289, Jeil Pharmaceutical will enter the POC trial in early 2016.
Patent applications covering materials and preparation methods were submitted in 2008 and approved in 2011 in Korea (10-0968175), as well as in the US, Europe, China, Japan, Australia, Canada, and Russia during 2011 and 2014.
- PCT: WO 2010/056038
Application for the JPI-289 crystalline structure patent was submitted in 2012.
In summary, one application in Korea and seven international applications have been registered, and registration processes for another application in Korea and seven international applications are currently underway.
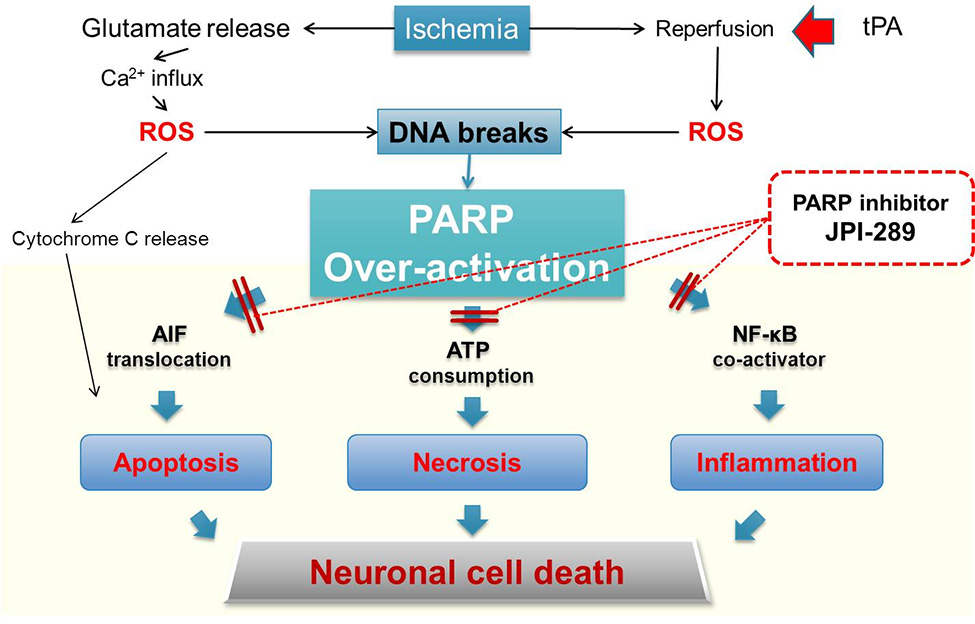
The majority of previous stroke candidates have been designed to inhibit apoptosis mechanisms; however, the clinical results have been inconclusive as most of the brain damages were caused by necrosis during the first 10 hours after the stroke occurs.
JPI-289 inhibits the damages caused by necrosis, apoptosis and inflammation, at the same time, and is expected to exert significant benefits in the treatment of stroke.
Competitive advantages
① Inhibition of PARP-1 is a significantly distinct mechanism of action when compared to other candidates and is expected to show high efficacy in clinical trials with ischemic stroke patients through the neuroprotective effects.
② Effective PARP-1 inhibition and the mechanism of action by MP-124 have been proven in a monkey model, which is the closest stroke primate animal model to human so far. In a monkey tMCAO stroke model, JPI-289 showed 49% decrease in infarction volume, which is the best result in the world when compared with that of 21% decrease in infarction volume by MP-124. Therefore, JPI-289 among PARP inhibitors is considered as one of the most promising agents for the treatment of stroke.
③ Safety of JPI-289 has been confirmed in healthy volunteers because there were no critical compound-related adverse events during phase IA study. AUCs of JPI-289 in the blood were increased dose-proportionally.
④ JPI-289 is highly soluble with excellent PK parameters. Single doses of JPI-289 significantly decreased infarct volume in an SD rat stroke model. Therefore, it is expected to be suitable for acute ischemic stroke patients who are required a prompt administration and onset of efficacy (JPI-289 iv infusion over 1 h vs MP-124 iv infusion over 24 h).
⑤ When JPI-289 was co-administered with tPA in the rat embolic tMCAO models, the infarction volume and hemorrhage area were significantly decreased compared to those of tPA single treatment group.
⑥ Because JPI-289 can be taken as an oral administration due to relatively high bio-availability (rat: 66%, dog: 100%) as well as an injection, treatment of stroke with JPI-289 after discharge is predicted to be maximized.
⑦ JPI-289 has shown excellent safety profiles in non-clinical studies, leading to IND approval for Phase I clinical trials in healthy male subjects. This is in favorable contrast to MP-124, another leading candidate that has been in Phase I since 2009. Acquisition of clinical POC via rapid completion of Phase IIA is expected to endow an advantageous position in licensing-out to global big pharmaceutical companies.
⑧ After secure of clinical POC, the drug value will be maximized by expanding its application for other diseases (e.g. myocardial infarction) and patient groups.
⑨ S100B, NSE will be scrutinized further to develop as a bio-marker of stroke in upcoming studies.
⑩ The synthetic process of JPI-289 has been established with DS/DP production in cGMP facilities for Phase I trials. As a result, mass production for commercial purposes is tangible.
⑪ A huge market value has been established with monopolistic right until 2029
CNS Disease (Stroke)
Feb. 01, 2015 - Nov. 30, 2015
Jeil Pharmaceutical Co., Ltd.
Phase 1b