[Press Release]
○ The KDDF experienced considerable success in the first year after its launch. The organization developed a domestic licensing-out project system and has built a global network to provide a stable innovative project selection system.
○ The KDDF, established in September last year through the joint support of 3 Ministries, including the Ministry of Education, Science, & Technology, the Ministry of Knowledge Economy, and the Ministry of Health & Welfare, has so far received a total of 65 research project applications, with 14 of these entering into contracts with backing and support.
○ Approximately 30 billion won in government funds was invested, of which more than 1.1 billion was directed to support clinical Phase 2 projects.
○ Concerning contract status, there are 7 projects with below candidate substances, 2 non-clinical cases, 3 clinical Phase 1 projects and 2 clinical Phase 2 projects, As for substances, synthetic substances are sought after most, with the highest number of research project applications at 5, all involving tumors (refer to Attachment 1)
○ With an aim to develop new drugs without delays, the KDDF leads a virtuous cycle connecting industry, academy, and research institutions, further strengthening its competitiveness.
○ KDDF supports the whole cycle of new drug development so as to avoid unnecessary waiting or delays in the attainment of exceptional outcomes in every phase through building candidate substances, non-clinical, clinic-integrated management systems, and also initiating networking among industry, academia, and research teams in terms of research phases.
○ In the Duksung University case, despite the nature of the basic research project to develop a candidate material, in the selection process, it succeeded in attaining 90% cash matching with 50% corporate matching, proving its competitiveness. Prof. Ae-Ri Moon stated, “The task of preliminary-phase research, due to its high-risk nature, can hardly be expected to have support from corporations, but the high soundness of the work was recognized by the KDDF, playing a major role in obtaining credit of up to 90% from corporate support”
○ Also, in the case of the research on ‘Long-acting Growth Hormone’ by Genexine, , there was a positive outcome of a technology transfer – joint research development contract with Handok Pharmaceutical Company.
○ The technology transfer – joint research development contract is regarded as a good success model in the field of new medicine, compared to the examples of the development after licensing out by previous business entities, which did not have the same success.
○ President Jun-Chul Kim (Handok Pharmaceutical CO., LTD) said,
“We evaluated the Genexine product as being a remodeled bio-beta product with global capability. This conclusion was made referencing the results of scientific work, transparent evaluation, and the selection process of the KDDF”.
○ In addition, the differentiated strategies such as the systematic advanced method of project selection, continuously upgraded evaluation tools, and the introduction of milestone based research periods, compared to other agencies, are being ascribed to KDDF competitiveness.
○ The KDDF is operated under the established rule to accept projects 6 times a year with the layout of milestone oriented research periods, and the accepted projects are to be processed as per document appraisal, presentation appraisal, site inspection, and investment review by teams of experts for the final contract. The evaluation tools being used at each respective stage of appraisal have been developed continuously through discussion by experts in various fields and phases of new drug development.
○ Worth noting, in terms of the systematic evaluation that the KDDF underwent, is that the KDDF’s pool of evaluators consisted of experts across all areas of diseases ranging from whole cycles to phases. The makeup of this panel ensured expertise and balanced perspectives.
○ Bi-monthly acceptance of applications has the advantage of allowing the applicant to apply for reassessment within a shorter period of time, which makes their research less likely to suffer delays due to down time, and provides more opportunities for researchers. Even the research projects that were not selected in the evaluation process are being provided with various consulting support.
○ The KDDF’s firm base has led to global expansion.
○ The Global New Drug Development forum held last May allowed for education on the ins and outs of getting drug permits through the participation of a large number of FDA officials from the United States,who said they appreciated having provided practical aid to Korean researchers in new drug development, while praising Koreans on the provision of a meaningful opportunity to build networks among KFDA experts and domestic researchers.
○ Subsequently in July, an MOU contract was established with PAREXEL, a global CRO. This marked the first global network established after the launching of the KDDF, forecasting strengthened professional consulting capability.
○ On September 13th, a special session will be provided by BIO KOREA where Foreign CRO management technique know-how will be conveyed to domestic corporations & researchers.
○ President DH Lee said “We made a successful technology transfer to a local firm one year after launching our operation. This fact displays other firms’ appreciation for our project competitiveness.” He added, “We are establishing a virtuous cycle of new drug development that can be said to be our biggest achievement”
○ At the same time, he said “We will strengthen our competitiveness by means of our objective and scientific selection, evaluation, and maintenance system to go beyond instituting a system to be stable,” and added “We will also strengthen our consulting role across all new drug development”
[Attachment 1] Status of Research Projects Filed/Contracted
[Attachment 2] Interviews of President Dongho Lee, KDDF
[Attachment 1] Status of Research Projects Filed/Contracted
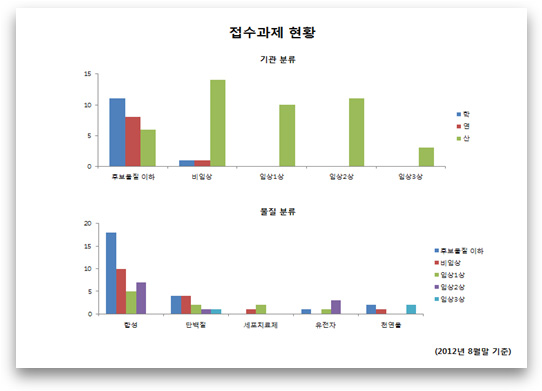
Title of Chart: Status of Research Projects Filed
a) Classification by Institution
Key (right-hand side): academy/research/industry
Key (horizontal axis): below candidate substance/non-clinical/clinical Phase 1/clinical Phase 2/clinical Phase 3
b) Classification by Substance Type
Key (right-hand side): synthetic/protein/cell therapeutics/gene/natural material
(As of August 2012)
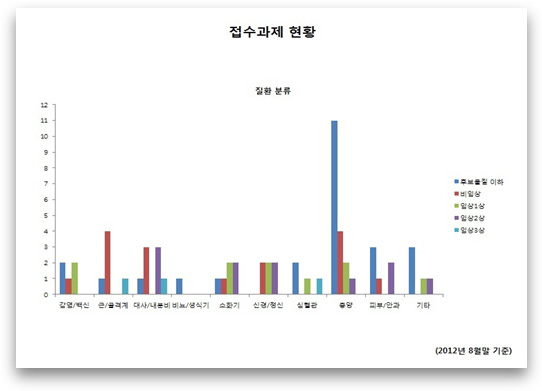
Chart Title: Status of Research Projects Filed
Classification by Disease
Key (right-hand side): below candidate substance/non-clinical/clinical 1st phase/clinical 2nd phase/clinical 3rd phase
Key (horizontal axis): infected/vaccine/metabolic/endocrine /digestive organs/ nerve/psychiatry
cardiac/tumor/ skin/ ophthalmology
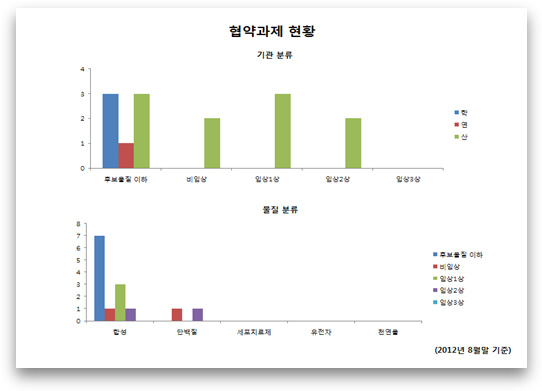
Chart Title: Status of Research Projects Contracted
a) Classification by Institution
Key (right-hand side): academy/research/industry
Key (horizontal axis): below candidate substance/non-clinical/clinical phase 1/clinical phase 2/clinical phase 3.
b) Classification by Substance Type
Key (horizontal axis): synthetic/protein/cell therapeutics/gene/natural material
(As of August 2012)
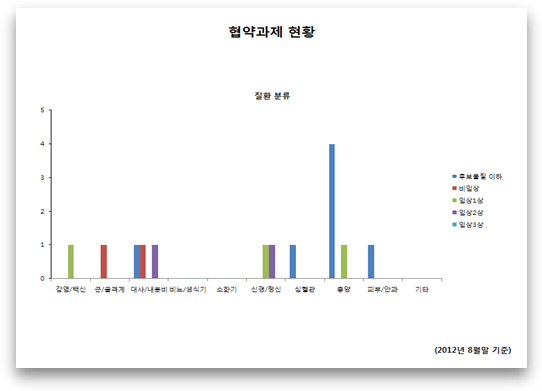
Chart Title: Status of Research Projects Contracted
[Attachment 2] Interviews of President Dongho Lee, KDDF
Q. What were your agency’s major accomplishments for the past year since launch?
A. For the past year we have succeeded in entering into 14 research project contracts after evaluation of a number of projects, including a local patient transfer. We have also brought about many other achievements by way of a pattern of business proceedings that differs from existing national R&D businesses. These accomplishments include the establishment of a foundation for international business networks, organizing international events, etc.
Specifically, the establishment of business promotion within a short timespan, the organizing of a pool of project evaluation experts, operational, organizational, and personnel management, and finally instituting our project management system can be quoted as major achievements that have strengthened our agency’s competitiveness.
Also, the new method of project selection occurring 6 times per year, escaping from the habitual year-length research period concept is thought to have increased the possibility of those projects having the potential to be developed as new drugs selected.
Establishment of a government R&D support system with such an innovative selection method including the milestone management system is a unique characteristic illustrating the competitiveness of the KDDF.
Q. How do you assess the project contracts for the past year?
A. We have entered into agreements totaling 14 research projects, which include 7 candidate substances, 2 non-clinical, 3 clinical 1st phase, and 2 clinical 2nd phases.
Of these, two research projects were under the 2nd phase milestone after having completed the 1st phase milestone successfully.
This indicates a balanced portfolio in terms of types of participating institutions, comprised of academies, research institutions, venture capital firms, small companies, medium-sized companies, and large corporations.
Most of the projects met or exceeded their goals through effective project management.
Judging by the results of project implementation in agency, development phase, and analysis of each substance based on the above, the foundation for long term success of our strategy appears to have been laid down, and I believe that a lot of solid effort was made strengthening seamless coordination between the R&D business and each government department and national infrastructure through the operation of research projects.
Q. What is the possibility of developing a new drug in Korea?
A. Compared to the past, nowadays overseas clinical trial planning applications are increasing and the number of new drug development researchers is rising, too.
I believe that active communication and cooperation among industries, academies, and researchers, as well as preparation of more flexible government support programs, will draw capable researchers into new drug development.
If a more selective, efficient, and reliable support system is operated intensively through KDDF for the full period of 9 years, we expect the appearance of various new drugs of blockbuster grade, or at least drugs that will work in niche markets.
It is very difficult to predict the exact potential, but looking at our excellent research manpower and national infrastructure support, I believe that our country will grow to become one of the new drug developing power house in the near future.
Q. What will be the role of the government that must be expanded for new drug development?
A. As you know, new drug development requires a lot of investment, and continued support by industry, academy, and research institutions appears as if it will be difficult.
Therefore, from the very beginning until the realization of profit, stable, systematic, professional support by the government is necessary.
Aggressive support is necessary by domestic researchers who can actively implement advanced foreign know-how and systems, while continuously increasing the R&D budget with more business oriented perspectives.
For example, making a priority of aggressive investing and focusing on project potential, with a standard allowing for the recovery of the money invested by the government needs to be provided, based on which the recovered funds will be recycled to maximize the efficiency of limited resources.
Q. What is the KDDF’s operational plan for the future?
A. To promote academic research capability, it is planned to proceed with the project incubation business. Also, a new drugs ‘re-creation’ project is expected to proceed, capable of developing new drugs in relatively short periods at lower costs, utilizing the excellent ideas of domestic researchers from the substances verified by global pharmaceutical companies overseas.
Also, through more active publicity, we will strongly encourage the submission of research projects to the KDDF that have more potential. For this purpose, we plan to hold promotional seminars as well. Specifically, we will act as a link matching those foundation’s researchers who have potentially-successful ideas but who lack funding for research expenses.
Also, for those researchers who lack experience in all phases of research, we plan to provide a consulting service through which we will assist for them in planning strategies that have the potential for success.
Moreover, we will not hesitate to provide our assistance in fulfilling these researchers’ goals as far as operations go.
Also, in order to boost the domestic capability of new drug development, we will continue to put our best efforts into fostering a group of experts for new drug development. To obtain favorable results, we will steadily provide opportunities for the introduction of research projects currently in progress to our foreign partners.